BrainVTA offers research grade, high quality AAV vectors with titers from 1 x10
12 to 1 x10
13 vg/ml. AAV vectors are prepared by eithe baculovirus infection in SF-9 insect cells or by a triple plasmid transfection in HEK-293 cells. Purified vector preparations are subjected to a variety of quality control measures including QPCR titer to asses viral genomes and silver stain to further assess titer and viral purity. Control stocks are assayed for Cre recombinase and Luciferase activity.
The advantages of AAV compared to other viral vectors
● Long-term gene expression. rAAV genomes do not integrate into the host cellular genome, it persist in the nucleus in episomal forms. As cells replicate and divide, these episome will be lost. However, It can be expressed continuously for more than 6 months in tissues where cell division is not vigorous.
● Strong diffusivity. Due to small size and higher titer, rAAV has much higher diffusivity than adenovirus and lentivirus. And AAV can cross the blood-brain barrier, it is an ideal tool for neuron and glial cell infection.
● Specific expression can be achieved. AAV has a wide variety of promoters and serotypes, This enables AAV to recognize and infect different organs and cells, which make AAV as the first choice for animal experiments.
● High safety. AAV has not been found to cause disease to humans, and it is the safest viral vector approved by the FDA in US that can be directly used in human for gene therapy.
● Low immunogenicity. When AAV is used to infect muscle, brain, eye and many others with local high dose, it is not easy to cause immune response;
●High stability. rAAV virus can be stored for 1 week at 4°C, and it is resistant to some reagents such as chloroform.
|
Viral vector |
AAV |
LV |
Ad |
|
Genome |
ssDNA |
ssRNA (+) |
dsDNA |
|
Coat |
Naked |
Enveloped |
Naked |
|
Type |
Non-integrating |
Integrating |
Non-integrating |
|
Infection |
Dividing and non-dividing cells |
Dividing and non-dividing cells |
Dividing and non-dividing cells |
Packaging
Capacity |
4.9kb |
6kb |
7.5kb |
Transgene
expression |
Potentially long-lasting |
Long-lasting |
Transient |
Immune
Response |
Very Low |
Low |
High |
|
Titer |
Up to 1012-13v.g/ml |
Up to 109TU/ml |
Up to 1012pfu/ml |
|
Expression abundance |
High-level expression |
Moderate to high level expression |
High-level expression |
|
Safety |
No pathogenicity has been found yet,
Has been approved by the EU and FDA,
Used as a carrier for gene therapy drugs |
No pathogenicity has been found yet,
It has been used in CAR-T therapy in the human |
May cause some coughing and runny nose |
Packaging service
Process of AAV packaging
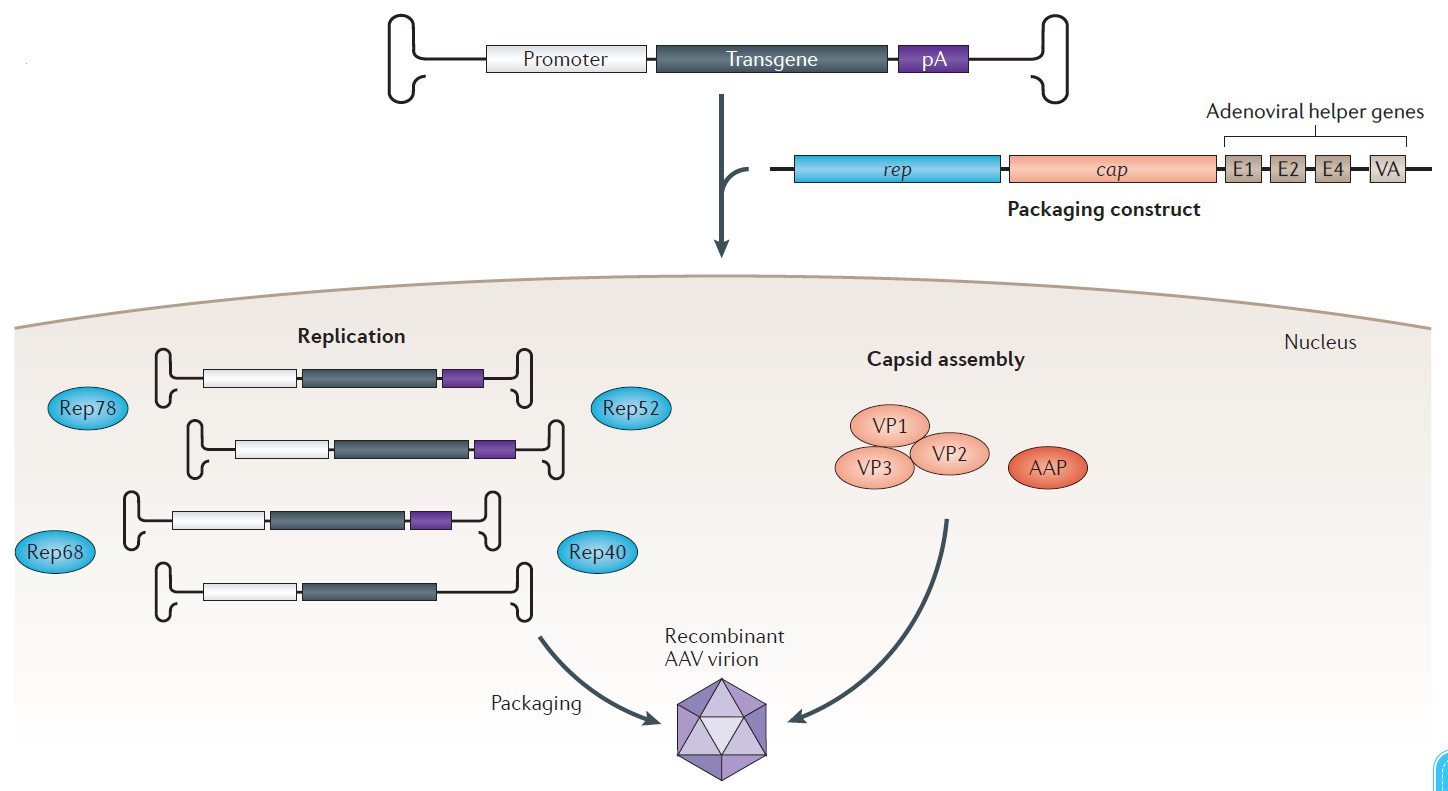 AAV virus packaging.(Melissa A. Kotterman.Nature Reviews et al.2014.)
AAV virus packaging.(Melissa A. Kotterman.Nature Reviews et al.2014.)
Step1: Clone the foreign gene into a suitable viral vector.
Step2: The recombinant expression plasmid is co-transfected into the AAV-293 cells with pHelper (carrying adenovirus derived genes) and pAAV-RC (carrying AAV replication and capsid genes), which supply the trans-acting factors required for AAV replication and packaging in the AAV-293 cells. Recombinant AAV is assembled in packaging cells 2 to 3 days after transfection.
Step3: Recombinant AAV viral particles are prepared from infected AAV-293 cells and may then be used to infect a variety of mammalian cells. Generally, AAV viral particles are enriched in packaging cells, so collecting cells and then lysing them to release AAV particles into the supernatant can recover most of the AAV viral particles.
Step4: Concentrate and purify the virus-containing supernatant above. The original supernatant contains many intracellular protein molecules and fragments. Purified virus is required for animal experiments, otherwise the required dose will not be achieved and side effects will be caused.
Step5: Quantitative PCR (qPCR) is one of the common methods to quantify the AAV vector titer. This method can obtain the physical titer of the AAV genome packaged in the viral particles. The infection titer of AAV varies greatly due to the infected cells, AAV coat protein and test conditions, and the experimental data in vitro cannot reflect the infection in vivo. Therefore, the physical titer obtained by qPCR is a more objective value.
How do I obtain virus packaging services?
● You can directly obtain Products from the spot list. Click here for more In Stock AAV Vectors Service.
● Also, You can obtain customized service using our highly intuitive free online vector design tool. To use the tool, click the
Design My Vector button. Once you finish designing a vector, you will have the option to put the customized service in your shopping cart.
● Lastly, If you are unable to use our online tool to design your desired vector or if you have other service needs, just submit your needs to us by clicking the
Send Request button. Our scientists will send you a link to a service proposal including price, turnaround and other relevant information. You can add the proposal to your shopping cart.
Virus serotypes
BrainVTA currently offers the following serotypes: 1, 2, 5, 6, 8, 9, rh10, DJ, DJ8,PHP.B, PHP.eB, PHP.S,retro,anc80. Novel peptide modified serotypes can also be produced on a case-by-case basis. Tissue specificity is determined by the serotype. If you Don't know which AAV serotype to use, See Table 1 to explore available AAV serotype to meet the efficient delivery of gene into the cells or tissues of interest.
Table 1 Target specificity of different AAV serotypes
|
Serotype |
Tropism |
|
AAV1 |
Muscle, Heart, CNS, Eye, Lung |
|
AAV2 |
In vitro, CNS |
|
AAV3 |
Human Liver Cancer Cells |
|
AAV4 |
CNS, RPE |
|
AAV5 |
Lung (airway, alveoli), Eye, CNS |
|
AAV6 |
Muscle, Lung, Heart, Adipose, Liver |
|
AAV8 |
Liver, Muscle, Eye, CNS, Adipose |
|
AAV9 |
Lung (alveoli), Liver, Muscle, Heart, CNS, Adipose |
|
AAVDJ |
In vitro, Liver, Heart, Kidney |
|
AAVrh10 |
Pleura, CNS |
Tips
● If you are not sure which serotype to choose, you can try AAV9.
● If you use AAV for the first time, you can use the Rainbow Colors AAV for pre-experiment. By which you can compare the infection effects of different serotypes on the target tissue, and explore the best injection method, injection site, virus dosage, etc., thereby getting more ideal experimental results.
Rainbow Colors AAV
Rainbow Colors AAV is a set product that combines common serotypes suitable for different organizations.
|
Rainbow Colors AAV |
Serotype |
Volume |
|
Eyes/other organs |
1/2/5/6/8/9/DJ |
10 μL/serotype |
|
Nervous system |
1/9/Retro/PHP. eB |
10 μL/serotype |
|
Liver/Kidney |
2/8/9/DJ |
100 μL/serotype |
|
Heart/Vascular/Muscle |
1/6/8/9 |
100 μL/serotype |
*AAV1 is used for anterograde tracer.
AAV9 is used as anterograde tracer.
AAVRetro is used for retrograde tracer.
AAV PHP.eB is suitable for crossing the blood-brain barrier.
Tissue-specific promoters
BrainVTA also offers various vissue-specific promoters. Listed below are the most commonly chosen promoters. Don't see the promoter of interest,
Please inquire.
Selection of vectors
BrainVTA provides a variety of AAV vectors, which can manipulate coding and non-coding genes through overexpression, RNAi and other technologies, such as lncRNA, microRNA, and circRNA. For more details, please contact
sales@brainvta.com.
