Hepatitis B virus (HBV) belongs to the Hepadanaviridae family. Which has highly infectious and stable through covalently closed circular DNA (cccDNA) in the cell nucleus of the host. The infection of HBV will result in acute or chronic necroinflammatory liver diseases. Moreover, chronic HBV infection is the major cause of hepatic cirrhosis and hepatocellular carcinoma (HCC), leading to high death rates. Although an effective vaccine was introduced years ago, the percentage of existing HBV carriers remains high and vaccination is not a treatment for established infections. As a result, effective animal models of HBV are indispensable for both mechanism studies and the development of novel therapeutic interventions.
BrainVTA developed rAAV8-1.3xHBV, that carries 1.3 copies of the full HBV genome. This product can realize the persistent infection of animal liver by intravenous injection so as to truly simulate the infection status of HBV and provide a powerful tool for the basic research of hepatitis B virus infection and the development of antiviral drugs.
Fig1.Genome structure diagram of rAAV-1.3xHBV, including 4ORF and upstream repeat areas of the complete hepatitis B virus genome
Product Features
● rAAV8-1.3xHBV carries the hepatitis B virus genome with ayw serotype and D genotype.
● rAAV8-1.3xHBV simulates the natural life cycle of the virus by the formation of pgRNA.
● A baculovirus-insect Sf9 cell production system is used, which contained no human cell lines, so it’s more safe.
● large-scale production and high yield and stability.
Effect Verification
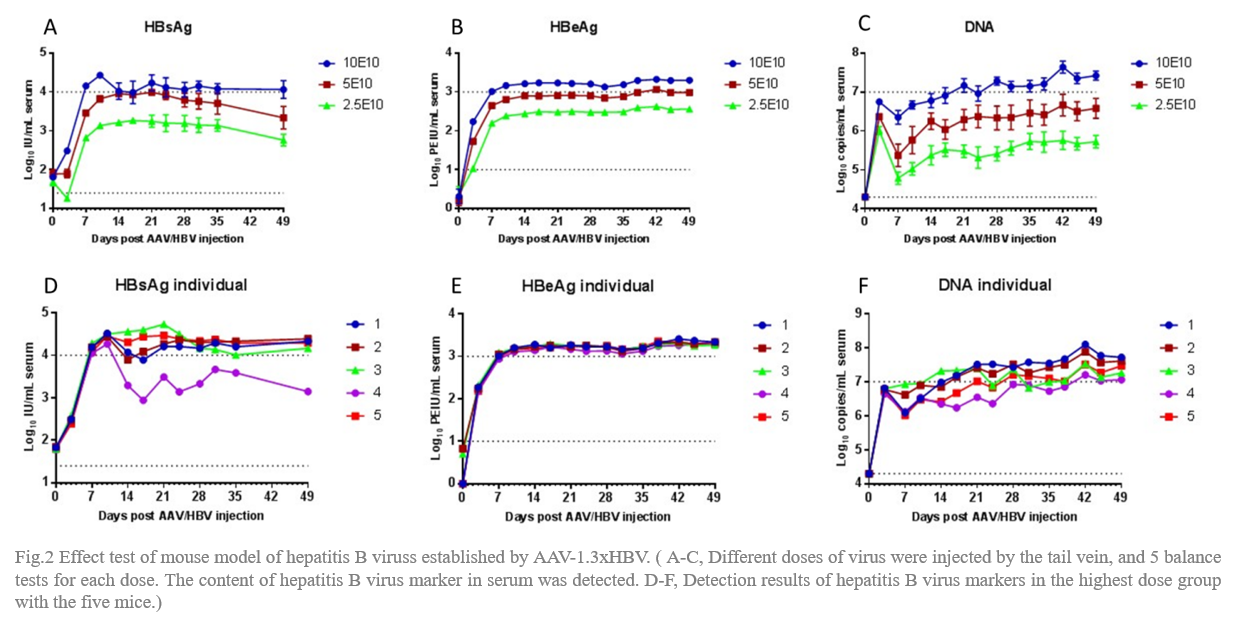
rAAV8-1.3xHBV was injected in C57 mice by via tail vein with the total virus is 2.3x1010vg, 5x1010vg and 10x1010vg, and the volume is 200uL, respectively. The contents of HBsAg, HBeAg and DNA markers of hepatitis B virus in serum were detected by orbital blood collection and observed continuously for 7 weeks.
Applications
● Establish of hepatitis B virus infection model
● Basic research on hepatitis B virus infection
● Development of antiviral drugs
References
1. Global Hepatitis Report, 2017. WHO
2. Chronic Hepatitis B Virus Infection: Developing Drugs for Treatment Guidance for Industry. 2018 FDA
3. Christoph Seeger, and William S. Mason. Hepatitis B Virus Biology. Microbiology and Molecular Biology Reviews (2000). 51-68.
4. Haifeng Wang, Seahee Kim, and Wang-Shick Ryu. DDX3 DEAD-Box RNA Helicase Inhibits Hepatitis B Virus Reverse Transcription by Incorporation into Nucleocapsids. Journal of General Virology (2009). 5815-5824.
5. Man-Young Cha, Dong-Kyun Ryu. Stimulation of hepatitis B virus genome replication by HBx is linked to both nuclear and cytoplasmic HBx expression. Journal of General Virology (2009). 90:978-986.

