COVID-19, a novel pathogenic coronavirus (2019-nCoV or SARS-CoV2) that causes the COVID-19 pandemic respiratory illness and spread globally rapidly. The SARS-CoV-2 (2019-nCoV) Spike Protein (S-RBD) has been identified as the key viral element allowing the virus docking to the ACE2 surface membrane receptor. So the SARS-COV-2(2019-nCoV) Spike Protein (S-RBD) is a key target for urgently needed vaccines, therapeutic antibodies, and diagnostics.
BrainVTA can provide SARS-COV-2(2019-nCoV) Spike Protein (S-RBD) with high-quality for small or large scale.
Pre-made products list
|
Cat.# |
Vector Name |
Price |
|
AC215-01 |
SARS-COV-2(2019-nCoV) Spike Protein (S-RBD), His Tag Recombinant Protein |
$300 for 100ug |
|
AC215-02 |
SARS-COV-2(2019-nCoV) Spike Protein (S-RBD), His Tag Recombinant Protein |
$300 for 100ug |
|
AC215-03 |
SARS-COV-2(2019-nCoV) Spike Protein (S-RBD), His Tag Recombinant Protein |
$300 for 100ug |
Product Details
Size 0.2ml
Concentration 1mg/ml
Class Recombinant
Type Protein
Form Liquid
Purification SDS-PAGE
Storage Buffer PBS, pH 7.4
Storage Conditions Store the liquid protein at -20°C or -80°C for long term, 4 ℃ for short-term storage.
Reconstitution Reconstitute to a new concentration in PBS. Repeated freeze-thaw cycles should be avoided.
Product Specific Information
• Molecular Weight: 29.13 kDa
• Animal Origin Free: Yes
• Protein Length: SARS-COV-2(2019-nCoV) Spike RBD fused with a 6×His tag at the N-terminus.
• Purity: 90%±5% by SDS-PAGE.
• Biological Activity: Measured by colloidal gold method. The activity was better and the detection limit was lower.
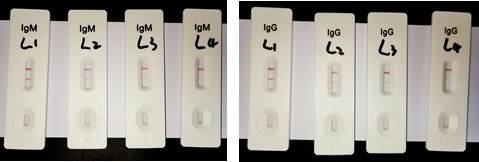
L1, L2 and L3 are respectively diluted samples of IgM and IgG positive samples ( L1: 256 fold dilution; L2: 512 fold dilution; L3:1024 fold dilution; L4: dilution buffer).
• Clinical verifications
The performance of the Dablood SARS-CoV-2 IgG / IgM Antibody Test was compared to a composite reference method that includes a RT-PCR method and a chemiluminescence immunosorbent assay (CLIA). Positive Percent Agreement (PPA) is 89.5%, the Negative Percent Agreement (NPA) is 99.7% and the Total Percent Agreement is 95%.
Applications
• Vaccination studies
• Antibody screening
• Inhibitor screening
• ACE2 cellular expression screening