COVID-19, a novel pathogenic coronavirus (2019-nCoV or SARS-CoV2) that causes the COVID-19 pandemic respiratory illness and spread globally rapidly. Due to its high pathogenicity and infectivity, live SARS-CoV-2 should be handled under biosafety level 3 (BSL-3) conditions, which has seriously hindered the development of COVID-19 vaccines and drugs. Combining the genomics and the biological characteristics of SARS - CoV- 2, BrainVTA has developed SARS-CoV-2 pseudovirus production system, from which the SARS-CoV-2 pseudotyped virus can be handled in biosafety level 2 (BSL-2).
COVID-19 Research Tools that BrainVTA can provide
●
shRNA, overexpression, Gene editor for the proteins that the virus interacts with, like ACE2, S, S1, E, M.
●
Pre-made SARS-CoV-2 pseudovirus particles encode luciferase, EGFP and mcherry
in lentiviral and
VSV.
● Pre-made AAV and lentiviral particles that
express S protein, TMPRSS2 and ACE2 receptor.
● Effector cells: human ACE2 overexpression stable HEK293T and BHK cell lines.
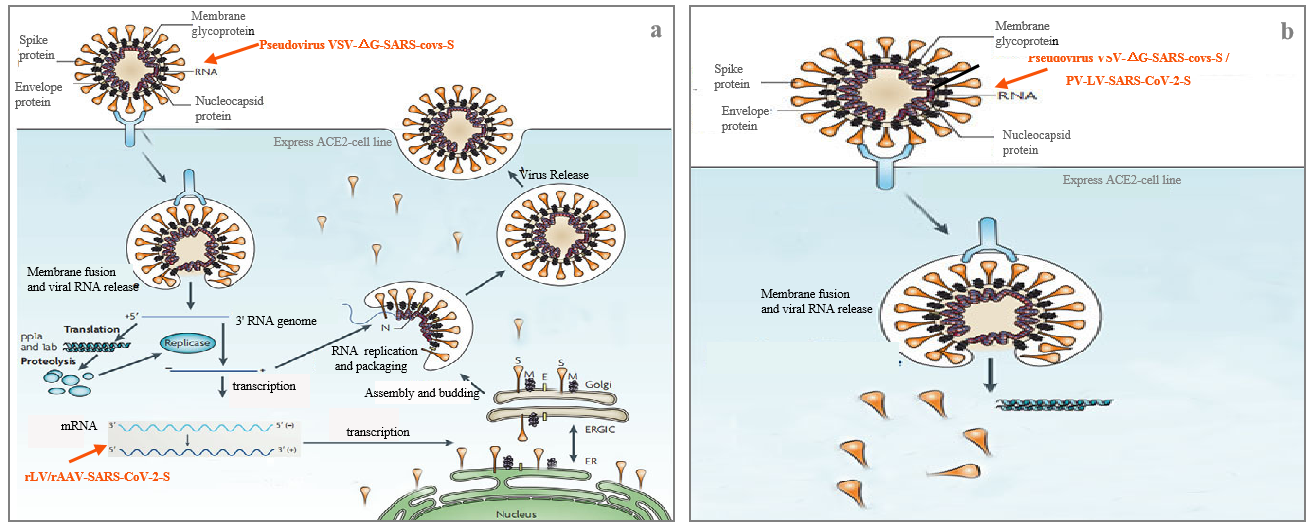 Fig.1 Schematic diagram of the pseudovirus simulating COVID-19 infection
Pre-made products list
Fig.1 Schematic diagram of the pseudovirus simulating COVID-19 infection
Pre-made products list
Table 1: SARS - CoV- 2-S protein packaged pseudovirus
|
Vector |
Cat. |
Vector Name |
Biosafety Level |
VSV vector
|
V04002 |
PV-SARS-CoV-2-S-VSV-△G-mCherry |
BSL-2 |
|
V04001 |
PV-SARS-CoV-2-S-VSV-△ G-EGFP |
|
V04003 |
PV-SARS-CoV-2-S-VSV-△ G-Luciferase |
Lentiviral vector
|
LV-0052s |
PV-SARS-CoV-2-S-LV-CMV-mcherry |
|
LV-0046s |
PV-SARS-CoV-2-S-LV-CMV-EGFP |
|
|
PV-SARS-CoV-2-S-LV-CMV-Luciferase |
Table 2 Recombinant viral express SARS-COV-2-S, ACE2 and TMPRSS2 protein
|
Vector |
Cat. |
Vector Name |
Biosafety Level |
|
Lentiviral vector |
LV-0537 |
rLV-CMV-SARS-CoV-2-S-2A-mCherry-WPRE |
BSL-2 |
|
LV-0538 |
rLV-Ef1a-ACE2-2A-EGFP-WPRE |
|
LV-0539 |
rLV-Ef1a-ACE2-2A-EGFP--CMV-Puro-WPRE |
|
LV-0558 |
rLV-CMV-TMPRSS2-2A-mCherry-WPRE |
|
LV-0559 |
rLV-CMV-ACE2-2A-TMPRSS2-2A-EGFP-WPRE |
|
AAV vector |
PT-2701 |
rAAV-CMV-TMPRSS2-2A-BFP-WPRE |
|
PT-2702 |
rAAV-CMV-SARS-CoV-2-S-2A-mCherry-WPRE |
|
PT-2703 |
rAAV-Ef1a-ACE2-2A-EGFP-WPRE |
Product Features
● PV-SARS-CoV-2-S-VSV-△G-EGFP/mCherry
1) As the genome of the pseudovirus can be replicate and the fluorescence signal will be enlarged, so pseudovirus in VSV has a high sensitivity.
2) Add PV-SARS-CoV-2-S-VSV-△G-EGFP/mCherry can only simulate the single infection process of the COVID-19, and if you want to simulate the infection continuously, the S protein needs to be compensated by infecting LV/AAV that express it.
3) It can be handled in biosafety level 2 (BSL-2) and the fluorescence expression time is 7-8h.
● PV-SARS-CoV-2-S-LV-CMV-EGFP/mCherry
1) Suitable for difficult to infect cells or tissues, such as stem cells, neurons, primary cells, etc.
2) Can only be used to simulate a single infection of COVID-19.
●Recombinant lentivirus express SARS-COV-2-S, ACE2 and TMPRSS2 protein
1) Suitable for difficult to infect cells or tissues, such as stem cells, neurons, primary cells, etc.
2) Suitable for the establishment of cell model.
3) Suitable for the establishment of effector cells.
●Recombinant AAV express SARS-COV-2-S, ACE2 and TMPRSS2 protein
1) Immunogenicity is low and suitable for long-term expression.
2) Suitable for the establishment of animal models.
SARS-CoV-2 Pseudovirus (PSV) Based Cell Entry
 Fig2. PV-SARS-CoV-2-S-VSV-△G-mCherry Based Cell Entry validation with fluorescence.
SARS-CoV-2(2019nCoV) Pseudovirus (PSV) Based Research Field
Fig2. PV-SARS-CoV-2-S-VSV-△G-mCherry Based Cell Entry validation with fluorescence.
SARS-CoV-2(2019nCoV) Pseudovirus (PSV) Based Research Field
●
Efficacy evaluation of therapeutic agents in the following antiviral mechanisms:
1)Inhibition of S protein binding to ACE2 receptor.
2) Inhibits virus release in intracellular vesicles.
● Analysis of neutralizing antibody levels
In the pseudovirus based neutralization assay, the inhibition of viral entry into cells by neutralizing antibodies is correlated to the decreased levels of luciferase or fluorescence (GFP and mcherry) signals in the ACE2 expression cells (HEK293T-hACE2 and BHK-hACE2).
1)To evaluate the effectiveness of therapeutic monoclonal neutralizing antibodies.
2)To analyze and evaluate the neutralizing antibody levels of COVID-19 vaccines during the development phase and after future marketing.
3)The dynamic change of neutralizing antibody level in COVID-19 convalescent patients.
● To construct a study model simulating SARS-COV-2 infected cells/animals.
 Fig.3 Establish the schematic diagram of cell/animal infection model
References
1. Jean K. Millet1, Tiffany Tang3,et al. Production of Pseudotyped Particles to Study Highly Pathogenic Coronaviruses in a Biosafety Level 2 Setting. J Vis Exp, doi:10.3791/59010 (2019).
Fig.3 Establish the schematic diagram of cell/animal infection model
References
1. Jean K. Millet1, Tiffany Tang3,et al. Production of Pseudotyped Particles to Study Highly Pathogenic Coronaviruses in a Biosafety Level 2 Setting. J Vis Exp, doi:10.3791/59010 (2019).
2. Nie J , Li Q , Wu J , et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2[J]. Emerging Microbes & Infections.2020.9(1):680-686.
3. Thomas T . SARS-CoV-2 receptor ACE2 expression in the human heart: cause of a post-pandemic wave of heart failure [J]. European Heart Journal.2020.9(1):45.
4. Wrapp , Wang NS, Corbett KS et al.Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation[J].Science.2020. 367:1260–1263



