Lentivirus (LVs) are single stranded RNA (sRNA) viruses, which are a subgroup of the retrovirus family. They can integrate into the host genome in both dividing and non-dividing cells, with a wide range of cell, resulting in long-term expression both in vitro and in vivo. The delivery vehicles based on HIV-1 have been developed by BrainVTA.
The advantages of LV compared to other viral vectors
● Long-term gene expression: Lentivirus can achieve long-term stable expression of target genes by integrating genes into the host cell genome, and will not be lost with cell division, which is the first choice for cell experiments.
● High safety. No pathogenicity has been found, and it has been used in CAR-T therapy in human;
● Low immunogenicity. Direct injection of tissues in vivo is not easy to cause an immune response and is suitable for animal experiments.
|
Viral vector |
AAV |
LV |
Ad |
|
Genome |
ssDNA |
ssRNA (+) |
dsDNA |
|
Coat |
Naked |
Enveloped |
Naked |
|
Type |
Non-integrating |
Integrating |
Non-integrating |
|
Infection |
Dividing and non-dividing cells |
Dividing and non-dividing cells |
Dividing and non-dividing cells |
Packaging
Capacity |
4.7kb |
6kb |
7.5kb |
Transgene
expression |
Potentially long-lasting |
Long-lasting |
Transient |
Immune
Response |
Very Low |
Low |
High |
|
Titer |
Up to 1012-13v.g/ml |
Up to 109TU/ml |
Up to 1012pfu/ml |
|
Expression abundance |
High-level expression |
Moderate to high level expression |
High-level expression |
|
Safety |
No pathogenicity has been found yet,
Has been approved by the EU and FDA,
Used as a carrier for gene therapy drugs |
No pathogenicity has been found yet,
It has been used in CAR-T therapy in the human |
May cause some coughing and runny nose |
Packaging service
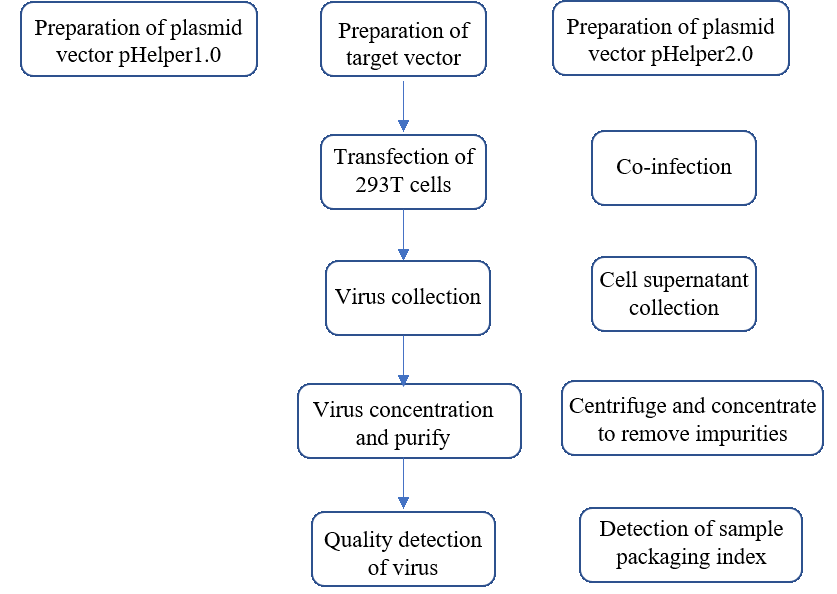
Step1: Viral vector preparation. Insert the target gene fragment into the lentiviral vector.
Step2: Co-infection in 293T cells. lentiviral plasmid and helper plasmid (Helper1.0 and Helper2.0)
Step3: Virus collection. The cell supernatant is collected by centrifugation after 48-72h of transfection.
Step4: Transformation and concentration. Process the cell supernatant by 0.45um filtration and ultracentrifugation.
Step5: Quality testing. The commonly used methods for titer testing are fluorescence, drug screening and absolute quantitative qPCR.
How do I obtain virus packaging services?
● You can click
Pre-Made LVs to find out whether the list contains what you need.
● Also, You can obtain custom-Made LVs service by clicking
here.
Characteristics of (HIV) Based Vectors
● Infects dividing and non-dividing cells;
● Long term, stable gene expression resulting from genomic integration;
● Pseudotyping with a VSV-G envelope glycoproteins that broaden cell tropism;
● Factors such as expression cassette size or choice of envelope will influence titer;
● HIV accommodates insert sizes of 6.5 kb. Larger expression cassettes can be packaged but decreases in titers will be expected.
Selection of vectors
BrainVTA provides a complete lentivirus product system, which can manipulate coding and non-coding genes, such as lncRNA, microRNA, and circRNA. For more details, please contact
sales@brainvta.com
Ready to order?
Further information about the lentiviral vectors, just click the
Pre-Made LVs or the
Custom-Made LVs Button and submit your needs to us.