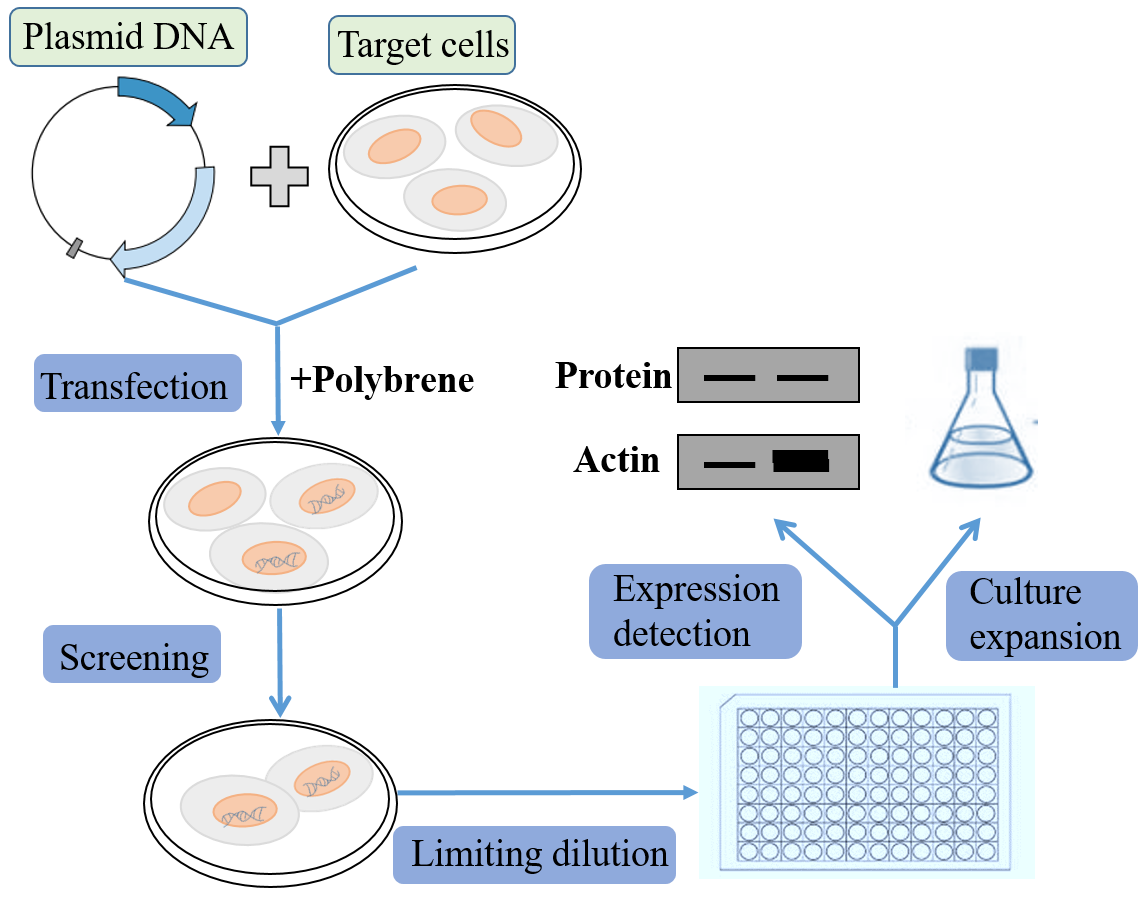
In contrast to transient expression, stable expression allows long term, as well as defined and reproducible, expression of the gene of interest, and facilitate long-term studies of genes on cell functions and protein interactions. As the mainstream stable cell line screening method, generating cell lines using lentiviral vectors overcomes the disadvantages of the long cycle of traditional plasmid used to pick monoclonal, and can efficiently obtain stable cell lines in a short time. The most commonly used resistance screening markers of eukaryotic expression vector include neomycin, hygromycin and puromycin. Cell lines that can stably express the target protein or specific silence genes can be obtained by further screening. Please note that not all lentiviral vectors deliver antibiotic resistance.
Stably-transfected cells can be selected by the addition of drugs to the medium, only if those cells which have integrated the plasmid survive, containing the drug resistant gene. Here we describe the neomycin resistance system, which uses resistance to G418 as a selection marker. Under various conditions, cells differ in their susceptibility to G418. The purpose of the preliminary experiment is to determine the optimal dose of antibiotics and to determine the lowest dose of antibiotics on selected cells. The specific experimental steps are as follows:
1. Cultivation of cell: Take cells in the logarithmic growth phase (untransfected cells, 70 % to 80 % of the bottom of the culture vessel), and use fresh antibiotic-free and serum-free medium to make 1×104 cells /ml of cell suspension. Inoculate the same amount into the multi-well culture plate and start adding medicine after 6 hours of cultivation(according to cell supplier instructions);
2. Screening with G418: determine several gradients in a range, and dilute G418 with medium according to the gradient concentration to make culture medium (e.g., titrate G418 in a range of 0.1 mg/ml to 1.5 mg/ml; in serum-free culture expand range down to 20 µg/ml), aspirate the medium, wash once with PBS, and feed cells with medium containing different concentrations of G418 to each well;
3. Change the medium: According to the cell viability and the color of the medium, change the screening medium every 2 – 3 days. If the cells grow too fast, the time for changing the medium can be shortened (every other day). Frequent replacement of the medium with dead cells can reduce the impact on viable cells;
4. Establish a death curve and determine the optimal screening dose: 10 to 14 days after screening, resistant clones can be seen, and the drug should be replaced with complete medium. If a large number of cells die, the G418 concentration can be halved to maintain screening.
Table1. Recommended concentration of antibiotics for screening(μg/ml)
|
Antibiotics |
Work scope(μg/ml) |
Common screening concentration(μg/ml) |
|
G418 |
50‐800 |
400‐500 |
|
Puromycin |
0.25‐10 |
0.5‐5 |
|
Hygromycin |
50‐800 |
200 |
● Infecting cells with lentivirus and screening stable cell lines
1. The 24-well dish will be spread at the appropriate cell density (around 80%) the day before infection.
2. Prepare lentiviral infection solution: remove the original medium and add a certain amount of MOI virus particles (the cell density during virus infection is 30 %-40 %).
Method for calculating the amount of expression vector added: (number of cells × MOI value/titer of virus stock) × 103 = amount of virus added (μl), cells must be fully covered by all medium.
Add polybrene: a. Polybrene is a polycation that can neutralize the charge to promote the interaction of the pseudo-virus shell and cell membrane. The optimal concentration of Polybrene varies with different cell lines and should be determined based on experience (usually in the range of 2-10 μg/ml). b. Exposure to Polybrene for a long time (> 12 hours) can have toxic effects on certain cells.
3. Replace the original medium with virus infection solution and continue to culture for 24 hours. Replace the viral infection solution with fresh medium;
4. 48 hours after virus infection, the transferred genes begin to express. At this time, the determined antibiotic concentration can be added to screen positive cells. This is the beginning of the selection process, which is to maintain the antibiotic concentration until the cells no longer die and the remaining cells grow normally.
5. In order to maintain the proper concentration of antibiotics and cell nutrition during the screening process, the medium should be changed every 2-3 days to remove dead cells and add antibiotics.
6. Evaluate cell lines in all aspects including stability test, batch test, and mycoplasma test.
If you have any questions,please email us at sales@brainvta.com.