With the development of viral gene delivery systems, many different viruses are being adapted as vectors to introduce genes into diverse types of cells and tissues, but the most advanced are lentivirus (LV), adenovirus (Ad) and adeno-associated virus (AAV). In this post, I will give a brief introduction to viruses and discuss some of their important characteristics to help researcher who will be using virus for the first time.
In general, researchers use viruses to deliver genetic material in vitro or in vivo, which is mainly used for 1) Gene overexpression; introducing a protein-coding gene into cells (using gne expression or gene editor) to study its function, or 2) knockout/knockdown; studying gene function through deletion or reduction of gene expression, respectively (using shRNA or gene editor).
Adenoviruses systems
Adenoviruses have a capacity of ~8.5 kb, which can infect both dividing and non-dividing cells with almost 100% efficiency. Unlike lentiviruses, adenovirus genomes and the foreign genes they carry do not integrate into the host cell genome, but are independently expressed outside the host genome, thus achieving transient and high levels of protein expression. The onset of expression can occur as early as 16-24 hours after infection. The high immune response from the target cells are the main limitation of adenoviral systems. Despite this, they are still widely used in research, due to their highly efficient transduction of most tissue.
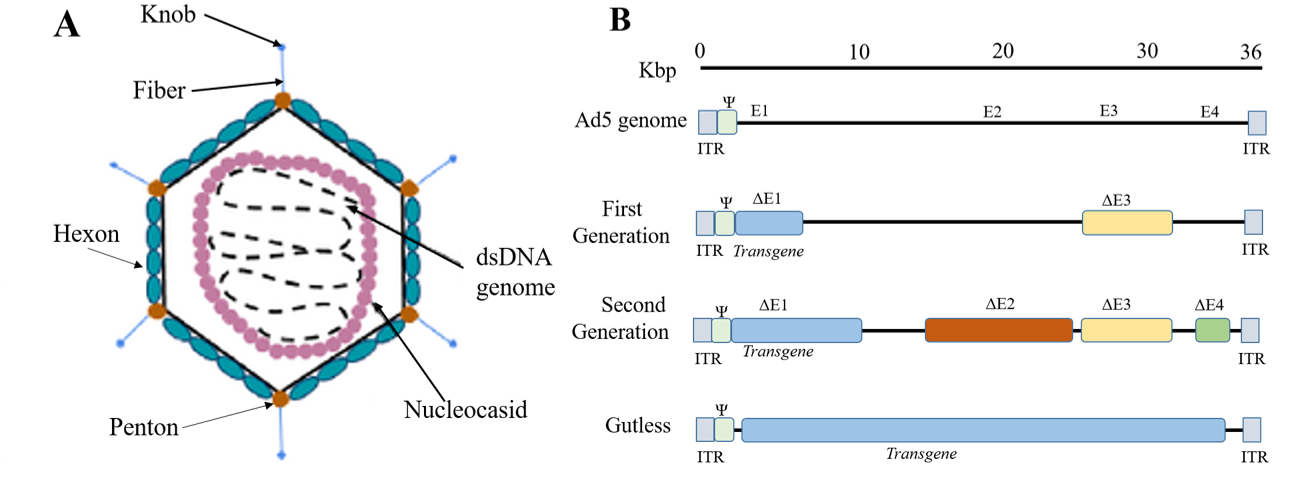
Figure 1. A)The structure of an adenovirus: the virion is ~100nm in size, and consists of an icosahedral capsid made of 3 types of proteins: fiber, penton and hexon proteins. The viral genome DNA is further protected by histone-like proteins forming the nucleocapsid. B)Three generations of adenoviral vectors: the first generation vectors are deficient in the E1 and E3 regions and can accommodate up to8.2 kb of transgene DNA. The second generation vectors are deficient in E1,E2,E3 and E4.Gutless Ad vectors (helper-dependent (HD-Ad, or high-capacity (HC-Ad)) are devoid of viral genes and therefore can accommodate up to 36 Kb. Gutless Ad maintain only the ITRs and the packaging signal(Ψ ), both of which are essential for assembly of the virion.(Adapted from Lee et al, Adenovirus-mediated gene delivery :Potential applications for gene and cell-based therapies in the new era of personalized medicine (2017))
Lentiviral systems
Lentiviral systems have been highly modified from HIV over several generations to make them safe to handle and useful for applications. At present, the third generation lentiviral system (Dull, et al. 1998), which requires four separate plasmids to produce infectious viral particles is often used for scientific research. Lentiviruses carrying GOI integrate into the genome upon infection, and can realize stable expression in both dividing and non-dividing cells. In addition, lentiviral particles carry the vesicular stomatitis virus G (VSV-G) glycoprotein in place of the wild type HIV env gene, which permit the high infectivity in a wide range of cell types, so they’re candidate for work with primary cells or other difcult-to-transfect cells. They are also commonly used to make stable transgene-expressing or knockout cell lines, or drive stable gene expression in organs and tissues in vivo. BrainVTA also developed the self-inactivating (SIN) Lentiviral systems, which contain a deletion in the U3 region of the LTRs. The self-inactivation vector prevents the integration to the tissues and cells. SIN Lentiviral systems are often used in stem cell studies to reduce the influence of foreign genes.
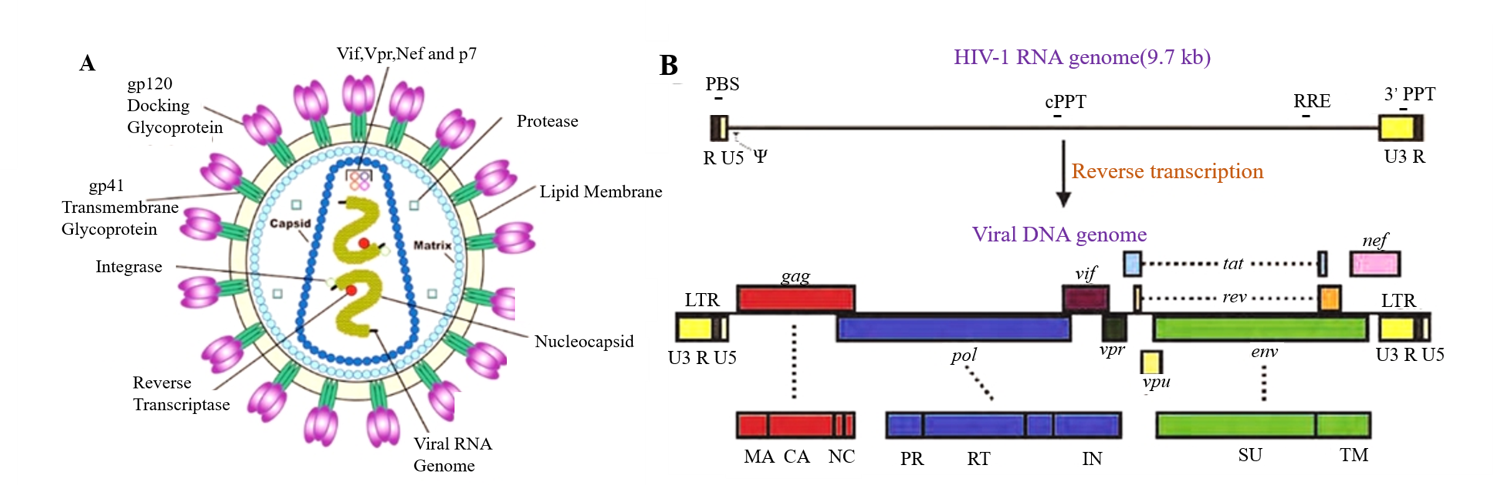 Figure 2. Schematic of the lentivirus genome and lentivirus-based vectors. (A) Capsid crystal structure. (B) The viral genome encodes structural, regulatory and accessory genes flanked by LTRs.
Figure 2. Schematic of the lentivirus genome and lentivirus-based vectors. (A) Capsid crystal structure. (B) The viral genome encodes structural, regulatory and accessory genes flanked by LTRs.
(Suzuki Y et al. Viral Gene Therapy. 2011.)
AAV systems
AAV has a single stranded DNA genome, which was discovered as a contaminant of adenovirus (Ad) preparations. Naturally-occurring AAV can integrates into the genome, but only at the AAVS1 locus on chromosome 19. The engineered AAVs can infect both dividing and non-dividing cells and persist in an extrachromosomal state without integrating into the genome of the host cell.
The recombinant AAVs have a packaging capacity of 4.5Kb, the potential for long lasting gene expression and comprises inverted terminal repeats (ITRs) at both ends of the DNA strand, and two open reading frames (ORFs): Rep and Cap. The primary disadvantage of AAV is its smaller packaging size for GOI, as well as a much later onset of expression (2-7 days for in vitro and 3-21 days for in vivo). However, AAV delivery system triggers very low levels of immune response.
 Figure 3. Adeno-associated virus biology. a: AAV Genome Structure b: Crystal structure of the AAV capsid(Melissa A. Kotterman et al. Nature Reviews Genetics. 2014.)
Figure 3. Adeno-associated virus biology. a: AAV Genome Structure b: Crystal structure of the AAV capsid(Melissa A. Kotterman et al. Nature Reviews Genetics. 2014.)
Further, AAV exists in different serotypes, which affect the tissue specificity. Multiple serotypes enable researchers to delivery gene specifically. All the most popular serotypes that list in the following table are available in BrainVTA.
Table1. The most popular used serotypes
|
Serotype |
Primary target tissue |
|
AAV1 |
Muscle, Heart, CNS, Skeletal muscle ,Neuronal and glial tissue |
|
AAV2 |
Eye,Muscle,Liver,neurons, CNS |
|
AAV5 |
Lung, Retina, Pancreas, Adipose, Liver, Neuronal and glial tissue |
|
AAV6 |
Muscle, Lung, Heart |
|
AAV8 |
Liver, Muscle, Eye, CNS, Adipose,Pancreas |
|
AAV-pan |
pancreas |
|
AAV9 |
Various, Retina, Lung , Liver, Muscle, Heart, CNS |
|
AAV.rh10 |
CNS、BBB、Pleura, |
|
AAV.DJ |
Various, Efficiently transduces a wide variety of cell types in vitro. |
|
AAV2/RETRO |
Retrograde |
|
AAV.PHP.S |
DRG、Heart、Colon |
|
AAV.PHP.eB |
Cross BBB |
Each type of virus has its own characteristics and can be used for specifc research applications. Details about the pros and cons of each virus are listed in the table below. If you have any questions, please feel free to contact at
sales@brainvta.com.
|
Type of Virus |
Advantage |
Disadvantage |
|
Lentivirus |
• Infects dividing and non-dividing cells, primary cell
• Broad tropism
•Long term stable expression |
•Insertional mutagenesis possible
•High immune response |
|
Adenovirus |
• Infects dividing and non-dividing cells
• Large packaging capacity
• High transduction efciency
• Broad tropism |
• Transient gene expression
• High immune response |
|
AAV |
• Infects dividing and non-dividing cells
• low immunogenecity
• Issue specificity |
• Transient gene expression
• Low packaging capacity (~4.5 kb) |
BrainVTA provide professional, convenient and R & D products experienced service for AAV, AD and LV, which help scientist accelerate the research process in circuit tracing, mechanisms and functional study.
Need a packaging services for virus?
We offer a wide range of viral vector services to suit your research needs.
AAV Production Service
Pre-made AAV center
shRNA Services
Genome Editing Service
Gene expression
Neuronal Tracing Tool Services
Is there something else we can help with? Check out these resources or feel free to contact us via email.
Introduction to AAV
Additional FAQ for AAV
Additional FAQ How to order
Additional FAQ for Custom Virus


