Recombinant adeno-associated virus vector (rAAV) is derived from non-pathogenic wild type adeno-associated virus and has the advantages of good safety and wide host range. rAAV has become a hotspot in gene therapy research, especially in the research of neurovascular diseases mechanism and treatment.
Vascular endothelium, as a functional tissue of human body, has the functions of barrier, synthesis, secretion and antithrombotic, etc. Studies have found that its dysfunction is closely related to the occurrence, development and prognosis of most cardiovascular diseases. It has been shown that intervention on key targets of endothelial cells can restore the function of vascular endothelium and thus have a certain therapeutic effect on cardiovascular diseases.
However, the infection rate of conventional serotypes such as AAV1, AAV2, AAV6, AAV8 and AAV9 on vascular endothelial cells was relatively low. To solve this problem, scientists developed a serotype that is highly effective at infecting endothelial cells by inserting specific short peptides into capsid proteins.
1. rAAVs serotype BR1 vectors highly infected with the brain microvasculature endothelial cell(Ref.1).
The rAAV vectors with the brain-targeted peptide NRGTEWD (BR1), unmodified wild-type AAV2 capsid, and the previously reported brain-targeting peptide DSPAHPS (PPS) was used, respectively, and then injected the rAAV virus into mice intravenously. After 4 weeks, the expression of luciferase reporter gene was detected by in vivo luminescence imaging of mice. The results are shown rAAV-BR1 vector displayed highly specificity and efficacy expression in the brain after inject in mice.
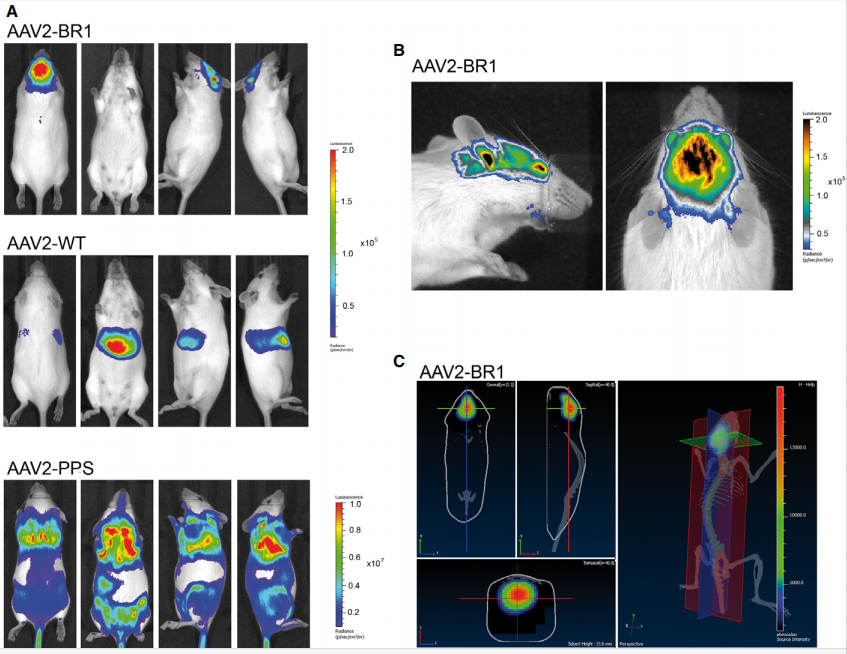 Fig.1
Fig.1 |
In vivo luminescence imaging of mice after intravenous injection of brain-enriched
AAV2 vectors carrying a luciferase reporter gene under the control of the CAG promoter. (Ref.1)
To identify the target cells of BR1-mediated transduction, the mice was treated with AAV2/BR1-CAG-EGFP by tail vein. Immunofluorescence staining(the endothelial marker CD31) of brain tissue from cerebellum, olfactory bulb, striatum, cerebral cortex, and the spinal cord were performed 14 days after vector injection. It’s shown BR1-eGFP-transduced cells (green) were positive with the endothelial marker CD31 (red). Therefore, AAV2/BR1-CAG-EGFP virus can efficiently infect vascular endothelial cells in cerebellum, olfactory bulb, striatum and cerebral cortex.
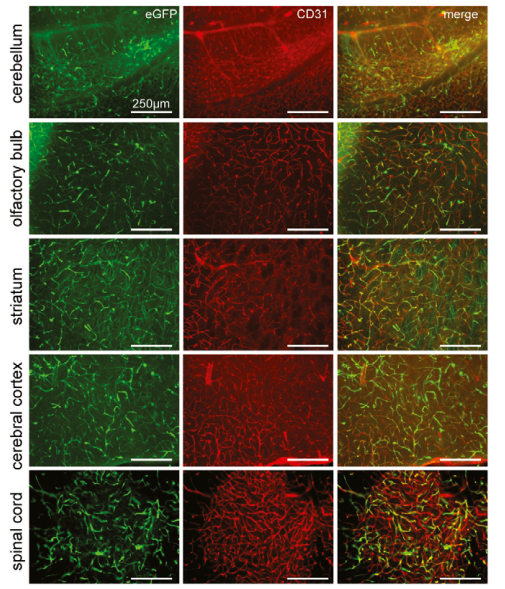 Fig.2
Fig.2 |
Representative images from cerebellum, olfactory bulb, striatum, cerebral cortex,
and the spinal cord, 14 days after vector injection. BR1-eGFP-transduced cells(green)
were positive for the endothelial marker CD31 (red).(Ref.1)
2. rAAVs serotype Vec vectors highly infected with endothelial cells (EC).(Ref2)
The rAAV vectors with the endothelial-targeted peptide VSSSTPR (Vec), unmodified wild-type AAV2 capsid, and the previously reported endothelial-targeted peptide NNPLPQR (Nec) was used, respectively, and then injected human umbilical vein endothelial cells (HUVEC) in vitro. The results showed that under the different gradient of the GOI, the efficiency of AAV2/Vec in infecting HUVEC cells was significantly higher than that of AAV2/Nec and AAV2/2, showing high specificity for vascular endothelial cells.
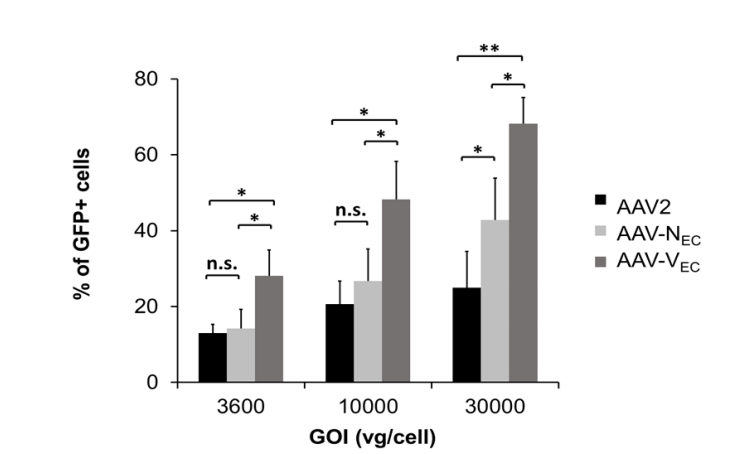 Fig.3
Fig.3 |
The AAV2/Vec, AAV2/Nec and AAV2/2 inject human umbilical vein endothelial cells (HUVEC) in vitro.
The adeno-associated virus with specific serotype can realize the targeted gene delivery, which greatly improves the efficiency of its infection to specific tissues.
BrainVTA provide the specific promoters and serotypes for CNS, muscle, liver, eye and other tissues for researchers which can meet various scientific research needs.
For more details, please email us at
sales@brainvta.com.
References
1. A brain microvasculature endothelial cell-specific viral vector with the potential to treat neurovascular and neurological diseases. Jakob Körbelin, Godwin Dogbevia, Stefan Michelfelder, Dirk A Ridder, Agnes Hunger, Jan Wenzel, Henning Seismann, Melanie Lampe, Jacqueline Bannach, Manolis Pasparakis, Jürgen A Kleinschmidt, Markus Schwaninger, & Martin Trepel. DOI 10.15252/emmm.201506078.
2. Capsid Engineering Overcomes Barriers Toward Adeno-Associated Virus Vector-Mediated Transduction of Endothelial Cells. L Zhang, A Rossi, L Lange, N Meumann, U Koitzsch, K Christie, M A Nesbit, C B T Moore , U T Hacker, M Morgan, D Hoffmann, J Zengel, J E Carette , A Schambach, A Salvetti, M Odenthal, H Büning. DOI: 10.1089/hum.2019.027.


