hM3Dq-mCherry was used for chemogenetical manipulation to activate the VTA
Nos1 neurons. AAV-taCASP3 was used to ablate VTA
Vglut2 neurons. GCaMP6f and GCaMP6s viruses were used for fiber photometry experiments.
The viruses used in this article are in the table below
|
Calcium sensors |
AAV2/9-CAG-DIO-GCaMP6f
pGP-CMV-GCaMP6s-EGFP |
|
Chemogenetics |
pAAV-hSyn-DIO-hM3Dq-mCherry
pAAV-hSyn-DIO-hM4Di-mCherry |
|
Optogenetic |
pAAV-EF1α -DIO-hChR2(H314R)-EYFP |
|
Neuron Ablation |
pAAV-EF1α -DIO-taCASP3-TEV |
Xiao Yu, Wen Li, Ying Ma, Kyoko Tossell, Julia J. Harris, Edward C. Harding, Wei Ba, Giulia Miracca, Dan Wang, Long Li, Juan Guo, Ming Chen, Yuqi Li, Raquel Yustos, Alexei L. Vyssotski, Denis Burdakov, Qianzi Yang, Hailong Dong, Nicholas P. Franks and William Wisden
Pub Date: 2018-12-17,
DOI: 10.1038/s41593-018-0288-9,
Email: [email protected]
We screened for novel circuits in the mouse brain that promote wakefulness. Chemogenetic activation experiments and electroencephalogram recordings pointed to glutamatergic/nitrergic (NOS1) and GABAergic neurons in the ventral tegmental area (VTA). Activating glutamatergic/NOS1 neurons, which were wake- and rapid eye movement (REM) sleep-active, produced wakefulness through projections to the nucleus accumbens and the lateral hypothalamus. Lesioning the glutamate cells impaired the consolidation of wakefulness. By contrast, activation of GABAergic VTA neurons elicited long-lasting non-rapid-eye-movement-like sleep resembling sedation. Lesioning these neurons produced an increase in wakefulness that persisted for at least 4 months. Surprisingly, these VTA GABAergic neurons were wake- and REM sleep-active. We suggest that GABAergic VTA neurons may limit wakefulness by inhibiting the arousal-promoting VTA glutamatergic and/or dopaminergic neurons and through projections to the lateral hypothalamus. Thus, in addition to its contribution to goal- and reward-directed behaviors, the VTA has a role in regulating sleep and wakefulness.
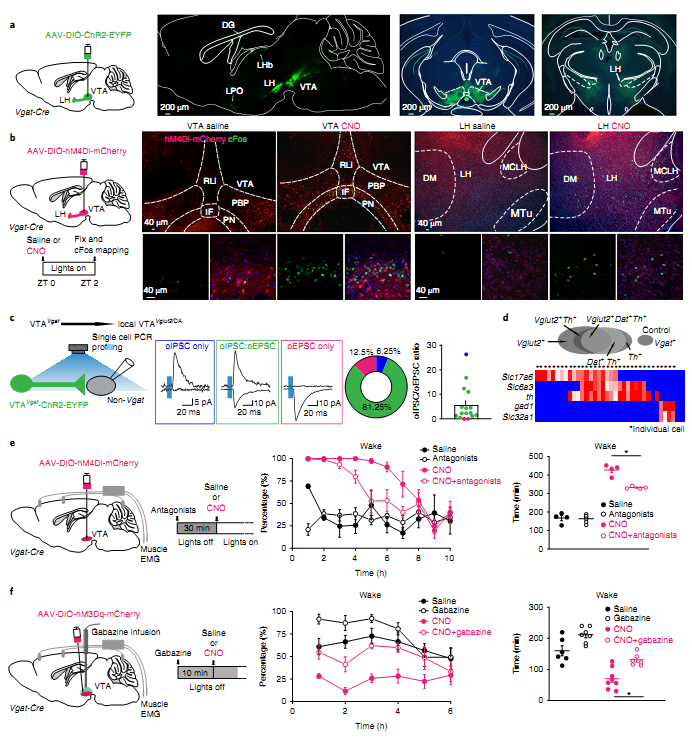 Figure. 1 VTAVgat neurons limit wakefulness in part by locally inhibiting dopamine and Vglut2 neurons in the VTA.
Figure. 1 VTAVgat neurons limit wakefulness in part by locally inhibiting dopamine and Vglut2 neurons in the VTA.
The authors describe a non-hypothesis-driven chemogenetic search for further circuitry controlling vigilance states, and unexpectedly converge on the VTA. In summary, the findings on VTA
Vglut2/Nos1 and VTA
Vgat neurons, and other recent discoveries on dopamine VTA neurons, identify the VTA as a critical center regulating wakefulness. The VTA is exceptionally well connected, receiving glutamate and GABA inputs from nearly all brain areas, making it well suited to serve as an integrator of vigilance state.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].
