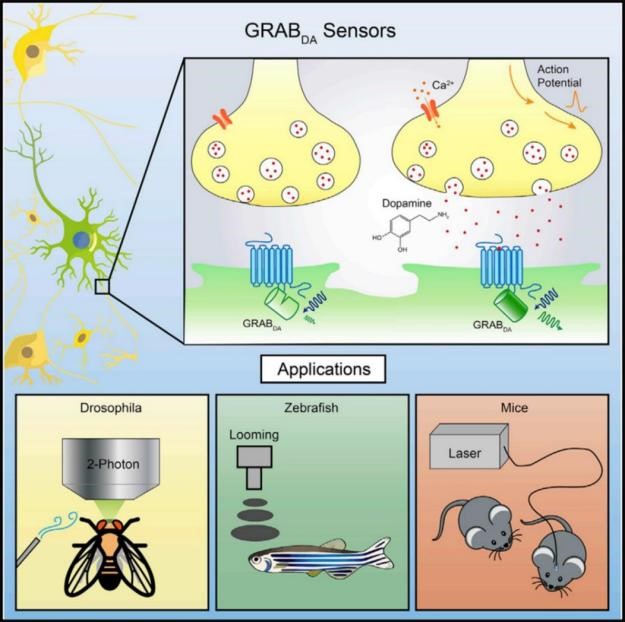
The complex function in the brain is inseparable from the efficient and specific information exchange and integration between neurons. Neurotransmitters (such as acetylcholine, dopamine, and neuropeptides ) are a key biological small molecules and participate in the process of information transmission mediated by chemical synapses between neurons, and are involved in a variety of physiological functions including development, information perception, motor regulation and higher-level cognitive behavior in the brain, and the devastating diseases will be caused once the malfunction of neurotransmitter, such as Parkinson's disease, Alzheimer's disease, addiction and epilepsy. Therefore, the study of neurotransmitter is essential for understanding the mechanism of brain function.
Sun, F., et al. Cell 2018
Li Yulong’s lab from Peking University develops cutting edge research tools, namely advanced imaging probes (GRAB sensors), including published dopamine (2018, Cell), acetylcholine (2018, Nature Biotechnology), and norepinephrine (2019, Neuron), to untangle the complexity of nervous system in space and in time, and helps to understand the changes of neurotransmitters in specific diseases, thus providing a new path for future precision medicine and new drug development.
The GRAB sensors could be expressed in specific cell-types and are able to detect dynamics of extracellular neurotransmitters in cultured cell, brain slice and living animal (e.g. fly, zebrafish, mice, rat, monkey etc.) by transfection, virus injection or construction of transgenic animals. For the aspect of instruments, GRAB sensors could perform well in epifluorescence microscopy, confocal microscopy, 2P microscopy and fiber photometry recording.
The Yulong Li Lab has deposited sensor plasmids at BrainVTA for distribution to the research community, and all GRAB
sensors (including the lastest ACh, DA, NE, 5-HT, Ado, ATP, VIP, CCK and eCB sensors) with AAV are available. To view the probe list, please click the
Neurotransmitter sensors or contact
sales@brainvta.com.
Note: please contact the Li Lab (yulonglilab2018@gmail.com) before ordering unpublished sensors.
Features of published sensors
|
Name |
Neurotransmitters |
Version |
Color |
Backbones(From human) |
Affinity |
Signal Response Amplitude |
Dynamics |
Downstream Signal Coupling |
Reference |
|
Ach2.0 |
Acetylcholine |
First generation |
Green |
M3 receptor |
EC50~1uM |
ΔF/F0~90% |
τon~200ms,
τoff~800ms |
Weak |
[1] |
|
Ach3.0 |
Acetylcholine |
Second generation |
Green |
M3 receptor |
EC50~2uM |
ΔF/F0~280% |
τon~112ms,
τoff~580ms |
Hardly |
[2] |
|
Ach3.0-mut |
Acetylcholine |
Control of Second generation |
Green |
M3 receptor |
EC50~0uM
(W200A mutation) |
ΔF/F0~1.8% |
/ |
/ |
[2] |
|
DA1m |
Dopamine |
First generation |
Green |
D2 receptor |
EC50~130nM
Medium affinity |
ΔF/F0~90% |
τon~60ms,
τoff~700ms |
Hardly |
[3] |
|
DA1h |
Dopamine |
First generation |
Green |
D2 receptor |
EC50~10nM
High affinity |
ΔF/F0~90% |
τon~140ms,
τoff~2500ms |
Hardly |
[3] |
|
DAmut(1st) |
Dopamine |
Control of First generation |
Green |
D2 receptor |
EC50~0uM
C118A and S193N mutation |
No effect |
/ |
/ |
[3] |
|
DA2m(DA4.4) |
Dopamine |
Second generation |
Green |
D2 receptor |
EC50~90nM
Medium affinity |
ΔF/F0~340% |
τon~40ms,
τoff~1300ms |
Little |
[4] |
|
DA2h(DA4.3) |
Dopamine |
Second generation |
Green |
D2 receptor |
EC50~7nM
High affinity |
ΔF/F0~280% |
τon~50ms,
τoff~7300ms |
Little |
[4] |
|
DAmut(2nd) |
Dopamine |
Control of Second generation |
Green |
D2 receptor |
EC50~0uM
C1183.36A and S1935.42N mutation |
No effect |
/ |
/ |
[4] |
|
rDA1m (rDA2.5m) |
Dopamine |
/ |
Red |
D2 receptor |
EC50~95nM
Medium affinity |
ΔF/F0~150% |
τon~80ms,
τoff~770ms |
Little |
[4] |
|
rDA1h (rDA2.5h) |
Dopamine |
/ |
Red |
D2 receptor |
EC50~4nM
Medium affinity |
ΔF/F0~100% |
τon~60ms,
τoff~2150ms |
Little |
[4] |
|
rDAmut (rDA2.5mut) |
Dopamine |
Control |
Red |
D2 receptor |
EC50~0uM
C1183.36A and S1935.42N mutation |
No effect |
/ |
/ |
[4] |
|
NE1m(NE2.1) |
Norepinephrine |
/ |
Green |
a2A receptor |
EC50~930nM
Medium affinity |
ΔF/F0~230% |
τon ~70ms,
τoff~ 750ms |
Uncoupled |
[5] |
|
NE1h(NE2.2) |
Norepinephrine |
/ |
Green |
a2A receptor |
EC50~83nM
High affinity |
ΔF/F0~130% |
τon ~30ms,
τoff~ 2000ms |
Uncoupled |
[5] |
|
NEmut |
Norepinephrine |
/ |
Green |
a2A receptor |
EC50~0uM
S5.46A mutation |
No effect |
/ |
/ |
[5] |
|
Ado1.0 |
Adenosine |
Control |
Green |
a2A receptor |
EC50~60nM |
ΔF/F0~130% |
τon~36ms,
τoff~1890ms |
Hardly |
[6] |
|
Ado1.0mut |
Adenosine |
|
Green |
a2A receptor |
EC50~0uM
F168A mutation |
No effect |
/ |
/ |
[6] |
|
5-HT1.0 |
Serotonin |
Control |
Green |
5-HT2C receptor |
EC50~22nM |
ΔF/F0~250% |
τon~0.2s,
τoff~3.1s |
Uncoupled |
[7] |
|
5-HTmut |
Serotonin |
/ |
Green |
5-HT2C receptor |
EC50~0uM
D1343.32Q mutation |
No effect |
/ |
/ |
[7] |
FAQ:
1. What do the abbreviation h/m/l/mut mean, and what about the number?
h/m/l is the abbreviation of high/median/low, which indicates the sensors' apparent affinity towards transmitter. Most of the GRAB sensors have transmitter-insensitive mutant for comparison with GRAB sensors to check signal specificity. The number represents the version of sensor, the higher version, the better performance.
2. How to use GRAB sensors?
GRAB sensors are genetically encoded sensors for neurotransmitters, similar to other genetically encoded sensors (e.g. GCaMP). They could be expressed in specific cell-types and are able to detect dynamics of extracellular neurotransmitters in cultured cell, brain slice and living animal (e.g. fly, zebrafish, mice, rat, monkey etc.). For the aspect of instruments, GRAB sensors could perform well in epifluorescence microscopy, confocal microscopy, 2P microscopy and fiber photometry recording.
3. Can the sensors be expressed in vivo for a long-term period?
Yes. We can record good signals from GRAB sensor after expression for 3 to 6 months in mice brain by AAV infection.
4. Can GRAB sensors detect excitability of neurons?
No. The change of fluorescent intensity only indicates the dynamics of corresponding neurotransmitter, which has no direct correlation with excitability of neurons. Other approach is needed for detection of neuron excitability.
Reference
1. Jing, M., et al. (2018). "A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies." Nat Biotechnol 36(8): 726-737.
2. Jing, M., et al. (2020). "An optimized acetylcholine sensor for monitoring in vivo cholinergic activity." Nat Methods 17(11): 1139-1146.
3. Sun, F., et al. (2018). "A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice." Cell 174(2): 481-496 e419.
4. Sun, F., et al. (2020). "Next-generation GRAB sensors for monitoring dopaminergic activity in vivo." Nat Methods 17(11): 1156-1166.
5. Feng, J., et al. (2019). "A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine." Neuron 102(4): 745-761 e748.
6. Peng, W., et al. (2020). "Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons." Science 369(6508).
7. Wan, J., et al. (2021). "A genetically encoded sensor for measuring serotonin dynamics." Nat Neurosci.